
Research Interests
Our research group primarily focuses on the stucture and dynamics of proteins in crowded milieu. The interior of our cells presents a very congested environment wherein there is a high concentration of different species. Since the latter occupy an appreciable volume inside the cell, thus they are known to exude what is commonly known as the "excluded volume effect", wherein the space occupied is not available to the protein under investigation. Earlier reports have already shown evidence of significant perturbation of protein structure and protein misfolding in the crowded environment. Our efforts are geared towards understanding the crowded milieu in a better way by employing a host of ensemble and single molecule based techniques, with fluorescence being the main spectroscopic tool.

Research Activities at a Glance
Enzyme Activity, Conformation and Dynamics

Understanding enzyme behavior in a crowded scenario through modulation in activity, conformation and dynamics
Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics
Volume 1869, Issue 11, November 2021,
In this study, we have investigated the manner in which the multidomain enzyme, AK3L1 (PDB ID: 1ZD8), an isoform of adenylate kinase, has its features affected in presence of commonly used crowders. Michaelis Menten plots reveal that the crowders in general enhance the activity of the enzyme. Ficoll 70, induced the maximum activity for AK3L1 at 100 g/L, beyond which the activity reduced. Ensemble FRET studies were performed to provide insights into the relative domain (LID and CORE) displacements. Solvation studies reveal that the protein matrix surrounding the probe CPM gets restricted in presence of the crowders, the same being linked to the decrease in the activity of the enzyme. Through our multipronged approach, we have observed a distinct correlation between domain displacement, enzyme activity and associated dynamics.
Correlating the Local and Global Dynamics of an Enzyme in the Crowded Milieu
Journal of Physical Chemistry B 126 (2022), 3208-3223
We have addressed the role of dynamic structural flexibility in affecting the activation energy barrier of a flexible multidomain enzyme adenylate kinase (AK3L1). Activation energy profiles of both local (at three different sites along the polypeptide backbone) and global dynamics of the enzyme have been monitored using solvation studies on the subnanosecond time scale and tryptophan quenching studies over the temperature range of 278–323 K, respectively, under crowded conditions (Ficoll 70, Dextran 40, Dextran 70, and PEG 8). This study not only provides the site-specific mapping of dynamics but reveals that the activation energies associated with these local motions undergo a significant decrease in the presence of macromolecular crowders, providing new insights into how crowding affects internal protein dynamics. The crowded scenario also aids in enhancing the coupling between the local and global motions of the enzyme. Moreover, select portions/regions of the enzyme when taken together can well mirror the overall dynamics of the biomolecule, showing possible energy hotspots along the polypeptide backbone.

Protein Conformation
Correlated and Anticorrelated Domain Movement of Human Serum Albumin: A Peek into the Complexity of the Crowded Milieu
Journal of Physical Chemistry B 120 (2015), 4897-4911
Using the multidomain serum protein HSA and a FRET-based approach, we have provided a detailed mapping of variations in the interdomain distances (as a function of pH) in presence of crowding agents. From the observation of correlated domain movements for dextran based crowding agents to anticorrelated motion induced by Ficoll 70, and both correlated and anticorrelated action for PEG8000 (PEG8), our results reveal the inherent complexity of a crowded milieu with the serum protein serving as an able sensor for decoding such variations. Differences in the manner in which the crowders of similar average molecular weights influence the protein conformational ensemble also provide insights into the possible variations at the molecular level that these polymeric molecules possess.


Unusual effects of crowders on heme retention in myoglobin
FEBS Letters 589 (2015), 3807-3815
We have investigated the unfolding of the protein myoglobin (Mb) entrapped in the confinement of the water pool of AOT reverse micelles and in the presence of some commonly used macromolecular crowding agents (Ficoll 70, Dextran 70, and Dextran 40). Confinement effects were found to be quite destabilizing in nature for Mb. Effects of the crowding agents on myoglobin also show a deviation from the general notion that synthetic macromolecular crowding agents are always stabilizing in nature.
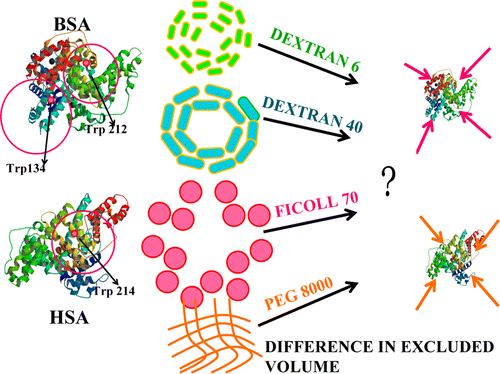
Crowding-Induced Quenching of Intrinsic Tryptophans of Serum Albumins: A Residue-Level Investigation of Different Conformations
Journal of Physical Chemistry Letters 4 (2013) 2610-2617
Used the sensitivity of the tryptophan (Trp) fluorescence of two homologous serum albumins (BSA and HSA) to its (Trp) surroundings to monitor the local changes in the immediate proximity of the intrinsic fluorophore. Through fluorescence quenching we showed that the commonly used synthetic macromolecular crowders (e.g., Dextran 6, Dextran 40, Ficoll 70, and PEG 8000) broiught about dramatic conformational modulations in the two proteins. The nature of perturbation was observed to be largely dependent on the specific crowding agent used. Additionally the extent of local structure modulation was found to be the largest either in the native state of the proteins or under near-native conditions.

Myoglobin Unfolding in Crowding and Confinement
Journal of Physical Chemsitry B 116 (2012), 12895-12904
We have investigated the unfolding of the protein myoglobin (Mb) entrapped in the confinement of the water pool of AOT reverse micelles and in the presence of some commonly used macromolecular crowding agents (Ficoll 70, Dextran 70, and Dextran 40). Confinement effects were found to be quite destabilizing in nature for Mb. Effects of the crowding agents on myoglobin also show a deviation from the general notion that synthetic macromolecular crowding agents are always stabilizing in nature.
Protein Dynamics

Influence of crowding agents on the dynamics of a multidomain protein in its denatured State: A solvation approach
European Biophysics Journal (2020) (Just Accepted)
In this work we have studied the dynamics of domain I of the protein human serum albumin (HSA) in its urea induced denatured states, in presence of a series of commonly used macromolecular crowding agents. HSA was labeled at Cys-34 (free cysteine) in domain I, with the fluorophore 6-Bromoacetyl-2-dimethylaminonaphthalene (BADAN), the latter acting as the solvation probe. In the partially denatured states (2 – 6 M urea), lower crowder concentrations (~ <125 g/L) induced faster dynamics, while the same became slower beyond 150 g/L of crowders. We propose that this apparent switch in dynamics is an evidence of crossover from the soft (enthalpic) to hard-core (entropic) interactions between the protein and crowder molecules. That soft interactions are also important for the crowders used here was further confirmed by the appreciable shift in the wavelength of the emission maximum of BADAN, in particular for PEG8000 and Ficoll 70 at concentrations wherein the excluded volume effect is not dominant.
pH dependent domain dynamics of HSA controlled by protein based crowding agents
Chemical Physics Letters B 688 (2017), 98-105
Here we have investigated the dynamics of domain I of human serum albumin (HSA) in presence of bovine serum albumin and lysozyme as crowders. 6-bromoacetyl-2-dimethylaminonaphthalene (BADAN) covalently attached to cysteine-34 of HSA was used as the solvation probe and changes in its solvation pattern in presence of the crowders were monitored as a function of pH. Lysozyme induced increased retardation of solvation while BSA brought about faster dynamics. Our observations reemphasize the importance of soft interactions even under conditions where repulsive charge-charge interactions dominate, thus reminding us of the enhanced level of complexity that the crowded milieu can possess.


Crowder-Induced Rigidity in a Multidomain Protein: Insights from Solvation
Journal Physical Chemistry B 120 (2016), 12501-12510
We have used solvation dynamics to monitor the changes in a specific domain of the multidomain protein human serum albumin (HSA) in the presence of various crowders. The solvation probe BADAN was site-specifically attached to the cysteine-34 of domain I of HSA. Analyses of the time-resolved Stokes shift of this probe in the presence of crowding agents revealed a significant retardation of the solvent coordinate, particularly in a crowder-dependent manner. We attribute the observed slowing primarily to the increased internal protein friction in the presence of these polymers, implying considerable stiffness of the protein matrix.
Protein Aggregation

Exploring the potency of the naturally occurring polyphenol curcumin as a probe for protein aggregation in crowded environments
International Journal of Biological Macromolecules 141 (2019) 1088–1101
We have used the naturally occurring polyphenol, curcumin, for probing the aggregation of the serum protein bovine serum albumin (BSA) in crowded environments. The distinctive spectral profile of this polyphenol in response to varying aggregating scenario is indicative of its high sensitivity towards detection of aggregates. We also gained possible insights into the heterogeneity of the aggregation mixture, by fitting curcumin's emission spectra to a sum of Gaussians in diverse aggregating conditions.
Crowder induced structural modulation of a multi-domain protein during its early stages of aggregation: A FRET-based and protein solvation study
International Journal of Biological Macromolecules 127 (2019) 563–574
Using FRET and solvation dynamics, we have tried to gain important insights into the structural rearrangements, during the early stages of aggregation of the multidomain protein BSA in presence of crowding agents. FRET studies show that there is an initial compaction in the domain size (domain I) at the early time points of incubation followed by an increase in the distance between the donor-acceptor pair. Solvent correlation traces of BADAN (labeled at free Cys-34 in domain I of BSA) reveal that the same domain becomes rigid during the initial phase of the aggregation process subsequent to which there was a gradual increase in flexibility, the latter we proposed being a necessary step that allows facile addition of more protein units.


Polyphenols in combination with β-cyclodextrin can inhibit and disaggregate α-synuclein amyloids under cell mimicking conditions: A promising therapeutic alternative
Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics 1865 (2017) 589–603
Examined the effects of four naturally occurring polyphenols in combination with β-cyclodextrin (β-CD) on the aggregation of α-synuclein in the presence of crowders. Even at sub-stoichiometric concentrations of the individual components, the polyphenol–β-CD combination(s) not only inhibited the aggregation of the proteins but was also effective in disaggregating preformed fibrils. Curcumin was the most efficient, followed by baicalein
with (−)-epigallocatechin gallate and resveratrol coming in next. Efficiency of curcumin results from a balanced composition of the phenolic OH groups benzene rings and flexibility. MTT assays on
cell viability showed that these combinations appreciably impeded the toxicity of the prefibrillar α-synuclein aggregates on the mouse neuroblastoma cell lines (N2a cells).
β-Cyclodextrin and Curcumin, a Potent Cocktail for Disaggregating and/or Inhibiting Amyloids: A Case Study with α-Synuclein
Biochemistry (2014) 53, 4081–4083
Aggregation of α-synuclein has been implicated in Parkinson’s disease (PD). While many compounds are known to inhibit α-synuclein aggregation, dissolution of aggregates into their constituent monomers cannot be readily achieved. In this study, using a range of techniques, we have shown that an optimized cocktail of curcumin and β-cyclodextrin, at appreciably low concentrations, not only inhibited aggregation but also broke up the preformed aggregates almost completely. We propose that these compounds exhibit synergy in their action and thus provide us with the exciting prospect of working toward the development of a suitable drug candidate for prevention and treatment of PD

